The standard immunosuppression protocol used to prevent rejection of a transplanted pancreas includes the use of steroids. Yet it is well recognized that there are unwanted side effects with steroid use (for example, increased incidence of osteoporosis, more stress on the insulin-producing islets in the pancreas, weight gain, cosmetic changes such as a rounded face, and thin skin and hair). As a result a number of transplant centers have begun to use non-steroidal immunosuppression protocols. But steroid-free protocols are still somewhat controversial and the risk of organ rejection caused by eliminating steroids, relative to the potential benefits of avoiding steroid-induced side effects are not well understood, nor well studied.
At the Transplantation Society conference in Miami this August, the University of Minnesota reported on the results of an in-depth study of patient and pancreas survival, organ rejection, steroid-related side effects, lipid metabolism, and quality-of-life for pancreas transplant recipients who were converted to a steroid-free immunosuppression protocol. Recently we reported favorable results from our preliminary analysis; this article for Insulin-FreeTIMES largely follows my presentation of the one-year results to the Transplantation Society in Miami in August.
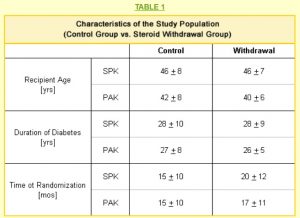
All of the patients in our study had received either a simultaneous pancreas kidney transplant (SPK) or a pancreas after kidney transplant (PAK) between five and 32 months before being selected for the study. All patients had full kidney and pancreas function; they were free of dialysis, and insulin-free with normal blood glucose levels. No patient had experienced a rejection episode for at least six months.
Twenty-six members of the study were PAK transplant recipients. Twelve patients continued to take steroids in the “control group,” and the remaining 14 were in the “withdrawal group” that converted to a steroid-free protocol.
We studied 30 patients who had received SPK transplants. Of these, 17 were in the withdrawal group and 13 were in the control group. This population was evenly divided between males and females.
Our study, therefore, included 56 people who had either a simultaneous pancreas kidney or a pancreas after kidney transplant. The twenty-five patients in the control group continued to take steroids, while the thirty-one patients in the withdrawal group converted to a steroid-free immunosuppression protocol.
The age, duration of diabetes, and the time since transplant when each member was included in the study are shown in Table 1. The average ages were similar for SPK and PAK recipients in both the control and withdrawal populations.